Introduction:
The earth is surrounded with the grip of fear of a microorganism named, COVID-19. It triumphs throughout all the continents in mother earth and is imperiling lives. In this situation of morbidity and fear, I as a chemist would like to emphasize on a subtle but powerful chemical bond that plays fundamental role to make life happens in this beautiful earth billions of years ago.
In a grime mist within the disc of the galaxy it is appallingly cold which causes to move “water molecules” very slowly. As soon as two slow moving clod water molecules meet each other, they get gluey together, it can be vehemently said that this stickiness is the harbinger of life in the universe, so it is worth understanding what the root cause of this stickiness is.
Hydrogen bond in water:
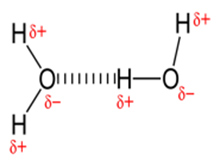
In a water molecule (H2O), due to the difference in electron attraction capability, the covalent bond (i.e. O-H) becomes dipolar, where partial negative charge concentrate on oxygen and partial positive charge develops on hydrogen atoms. Consequently an electrical attraction between these dipoles of different water molecules develops an electrostatic attraction as shown in the picture and hydrogen bonding occurs. Hydrogen bonding is directional and of electrostatic in nature and is the reason for stickiness.
Molecular processes of life:
It is known to us that ‘Life’ adopts various levels of complexity both structural and functional to reach from molecular levels to supramolecular levels which are capable of performing numerous functions. Leaving this evolutionary outcome which is impulsive, in this writing we shall emphasize on the interactions that take place between biomolecules are facilitated by well-known chemical bonds and forces that obey the universal laws of physics and chemistry.
Leaving all the enunciating list of properties to define life if only concentrate on very basic properties those are minimum requisite which must be justified as prerequisite of life to start the molecular process are as follows:
- i) Genetic and catalytic tasks performed by functional molecules/groups to sustain life:
Here genetic tasks imply exchanging information which are coded sequentially by chemical groups and catalytic processes reduce the barriers of several chemical reactions to synthesize molecular constituents. Consequently, molecular replications occur, which are vital criterion of molecular process of life. Nucleic acids, proteins and RNA are doing these tasks to preserve basic molecular constituents to continue life for billions of years.
- ii) Advent of molecular constituents with genetic and catalytic capabilities through the process of natural selection.
Any form of life which satisfies above two prerequisites must possess a variety of functional groups consisting of a huge number of atoms to store genetic information as well as catalytic processes for their evolution. Topology of such chain of functional groups ( a very large sequence of atoms) must be able to take any shape instead of linear. To pass the information from one group chain to another they must interact with one another.
Functional group chain and their interactions:
The existence of these “functional group chain” infers that adjacent chemical groups along the chain must be held together by chemical bonds which leads to a specific structure maintaining their atomic sequences. Moreover, along with these intra-chain bonds, to continue genetic tasks of copying and translating information, the chains of the functional molecules are expected to undertake molecular association (or dissociation) that comprises of sequential pairing (or un-pairing) of a series of chemical groups located along adjacent chains. Thus molecules in chain will be able to attain required conformations through folding, which is an intramolecular form of bonding. when the functional molecules interact with each other must be directional because the molecules involved bonding interactions must recognize one another other before accomplishing functions like catalyze chemical reactions, synthesis or recycle molecules/functional groups, exchange information among several functional group chains. These directional interactions, needed for intermolecular recognition, plays pivotal role of genetic exchange of information. To preserve the conformation and functionality of the group chains, the energy exchanged during these intermolecular bonding as well as recognition, must be lower than the inter-chain bond energy.
Till now it is comprehensible that intra-atomic bond among atoms to build functional group chain requires highest value of dissociation energy while intermolecular interactions requires for chain pairing for genetic information exchange requires somewhat less stronger bond than earlier but must possess directionality in its characteristics.
Roles of Chemical Bonds:
Covalent, ionic, and metallic bonds are the three principal forms of chemical bonds in nature. In covalent bonding adjacent atoms share their electrons in common orbitals; since the geometry of the orbitals creates optimum bond angles, covalent bonding is very directional. However, ionic bond arises from the electrostatic attraction between opposite charged ions are less directional than covalent bonds. Metallic bonds form due to electrostatic attractive force between free conduction electrons and a lattice of positively charged metal ions and also do not possess directionality. Therefore, covalent bonding is the feasible to form intra-atomic functional group chain but is not suitable to form inter-chain pairing or un-pairing interactions since the very nature of sharing of electron pair in common orbital in case of covalent bond would lead to formation new but unwanted electronic orbitals among inter chains, altering the atomic sequences of the chains, contrary to the constraint that the functional molecules must not be precious by inter-chain interactions This would lead to destruction or distortion of information going to be shared. This non-invasive nature of bonding can only be satisfied by weak forces of interactions like hydrogen bonding, halogen bonding, van der Waals interactions. However, van der Waals interactions are too weak to sustain in various conformation and also it is non-directional. Halogen bonds can be characterized similarly to hydrogen bonds in terms of nature of bonding and directionality but the larger size of halogens (Cl, Br, I) will cause steric hindrance which will restrict torsional bond angles to adopt any conformation while packing.
Hydrogen bonds: the major player:
From the above discussion, it is obvious that hydrogen bonding is the best option to carry out inter-molecular interactions among chains of functional groups. As mentioned earlier that, hydrogen bonds are directional in nature and this directionality rises with increase in its strength (by changing electronegative partner as well as distance between two dipoles) because the stronger bonds are more difficult to distort. Due to small size of hydrogen, the hydrogen bond shows orientation effect which is very important prerequisite for molecular recognition. Transfers of protons through hydrogen bonds are often coupled with electron transfer processes which are essential molecular processes to maintain neutrality in biochemical systems. At pre-biotic condition, due to the presence of high energy irradiation, hydrogen bonding might also played a medium for extended proton delocalization both in ground state and excited state following absorption of photons are absorbed. In conclusion it can be claimed that the prebiotic processes which initiate genetic and catalytic activity among functional groups with a significant and extensive presence of hydrogen bonding
Linus Pauling quoted regarding hydrogen bond “It has been recognized that hydrogen bonds restrain protein molecules to their native configurations, and I believe that as the methods of structural chemistry are further applied to physiological problems it will be found that the significance of the hydrogen bond for physiology is greater than that of any other single structural feature.”
At last I would like to conclude that the COVID-19 has given us a boon to redefine our mental bonding among us irrespective of the boundaries of nation, caste, creed etc.in terms of being human with sense of fellow feelings with each other. Mental bonding among us, voice of sanity must flourish the life of people as well as the planet in equal measure after overcoming this pandemic situation. .
References:
1.Hydrogen Bond and life in universe by Giovanni Vladilo , ID and Ali Hassanali, Life, 2018,8,1
- Requirements and limits for life in the context of exoplanets C.P. McKay,. Proc. Natl. Acad. Sci. USA 2014, 111, 12628–1263
Visited 1759 times, 3 Visits today



