Human mind always thrived to venture the uncharted territories of unknown. Tiny constituents of matter, energy and their relation with cosmology are ventured by enthusiast of Particle Physics. Investigations are carried out to probe deeper into the structure of matter in order to seek at every stage the constituents of previously so called ‘Fundamental Entity’. In our search of ultimate structure of matter, it was only known that the atom is basic constituents of matter and it is electrically neutral as a whole.
Atom Model:
The quest for fundamental particle inspirited by J.J. Thomson to look inside the atom with the discovery of electron in 1897 and an era of fundamental particles began. Thomson conception of atom- first suggested in1903. He imagined that atom consists of positive sphere of electrification within which were the negative electrons where all positively and negatively charges are uniformly distributed within a sphere of radius ~10-10m making the atom electrically neutral.
This Plum Pudding model of Thomson is challenged by Rutherford by his pioneering experiment of large angle scattering of Alpha particles (α) by thin gold foil in 1911. Rutherford showed such conception of atom model could not possibly account for the number of alpha particles scattered through large angles in Geiger and Marsden’s experiment. Alpha particle (2He4) is a positively charged particle and the particle suffers an intense repulsive electrostatic force and found to scatter almost at an angle more than 1500. The core of atom is alleged to consist of a minute positively charged nucleus which provides the intense electric field. Under the action of central inverse square law of force of repulsion, the path of α particle will be a hyperbola with nucleus as external focus.
A plot of the scattering angle vs number of scattered particle shows that the positively charged particles (protons) are concentrated at a core called nucleus. Thus suggesting the fact that ‘the atom has structure’! He also showed that the effect of the electrons outside the nucleus is negligible for deflection of α particle more than 10. Rutherford succeeded in estimating the size of the nucleus subsequently. He also discovered proton in 1919.

Long before the ninetinth century it was known from experimental observation that the elements emit line spectra and there are similarities in appearance of the spectra of different alkai metals. Some genuine explanation on these series spectra were extended by Balmer, Rydberg and others but there was little idea of the nature of mechanism in the atoms responsible for emission of spectral lines of characteristic fequencies. In 1900 Planck explained the law of blackbody radiation postulating that radiation of energy is not emitted or absorbed in cotinuous manner but in the form of discrete ‘Quanta’ of energy of magnitude ‘hν’ where h is Planck’s constant.
The first concept of quatum behaviour of nature. This discrete quanta is now known as photon named by G.N Lewis later on. Bhor has used all these concept to explain the spectrum of hydrogen. His postulates are based on discrete nature of atomic spectra in particular certain spectral lines of Hydrogen spectra in visible range (Balmer Series). In 1913 Bohr put forward his model for atoms with his postulates where the negatively charged electrons are suggested to be moving around the nucleus in some quantized stationary orbits. If an electron made a quantum jump from a statinary orbit, it will radiate frequency ν of energy hν. He is the key figure for driving the subsequent theoretical development of Quantum Mechanics. His explanation for line spectra may be considered as the first triumph of Quantum Dynamics too. With Bohr postulates we come across a picture of an atoms with nucleus at the centre and electrons moving around in quantized orbit.
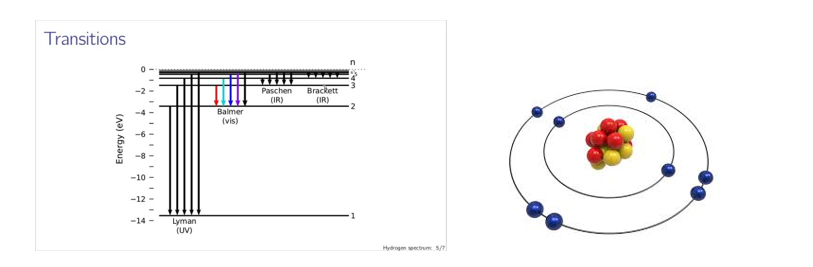
The complete picture of an atom was yet to be obtained. The Helium nucleus is found to be more massive than the prediction. Scientists were sure about the fact that something is missing with the discrepencies between mass number and atomic numbers. In the year 1932 James Chadwick discovered a particle named neutron from the Cavendish Laboratoty. His discovery comes from the apparant incompatibility of the energy momentum conservation of bombarment of Beryllium by alpha particle.The discovery of neutron completely reshaped the lanscape in the field of Nuclear and Particle Physics.
Deep Inelastic Scattering Experiments (DIS) and Quark Model:
In our quest for atomic structure we come across three elementary particles. Electron which is a Lepton (small mass), proton and neutron. Elementary particles are the particles to which no internal structure can be assigned. But much more surprises were waiting for physicists as more experimental results on scattering at much higher energy started to arrive.
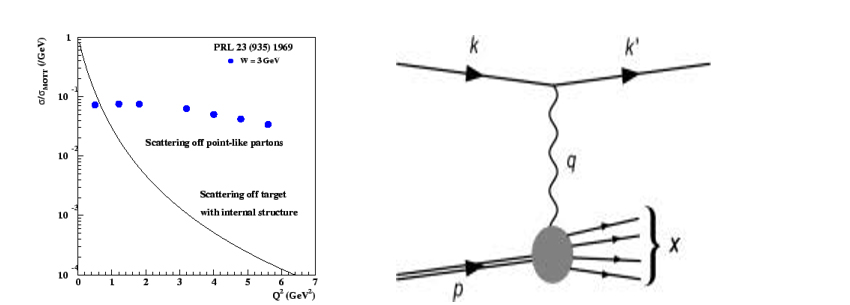
In high energy accelerators, electrons are accelerated at very high energy and allowed to scatter from a stationary proton. Proton gets disrupted due to high energies and produce various new particles. In such an inelastic scattering, the scattering cross section is found to drop abruptly while over all cross section of events producing the other particles remains almost constant. This type of scattering guides us to the recent depiction of proton. This experimental result suggests that proton possess three point of deflection and it has structure!
In order to search for the sub structure of hadrons (baryons like proton and also mesons) Gell Mann and Zweig put forward their Eightfold way in 1961 and Quark hypothesis. They have suggested that all baryons and mesons are composed of a much fundamental ‘entity’ of fractional electric charge which they named Quarks. Baryons are supposed to be three quarks
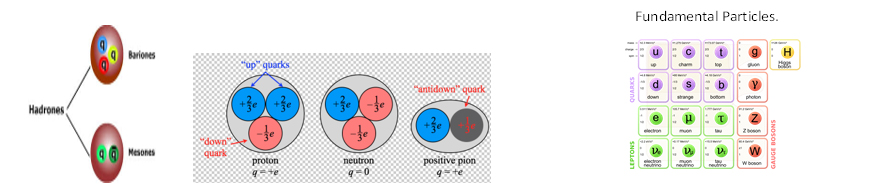
system whereas meson is quark anti-quark system. They first proposed the existence of fractionally charged particles. Initially three types of quark flavour are suggested (up, down, Strange). Proton consists of three quarks namely two up(u) quarks, a down quark (d) whereas neutron consists of two down quarks and a up quark. Subsequently more three type quarks are suggested making the number of quark flavour six in total to account for the structure of zoo of subatomic particles discovered later on. Six types of leptons are known. Probably the Quark-Lepton symmetry of nature will restrict the number of quarks upto six although QCD, theory of strong interaction predicts more types of quarks theoretically. So the Fundamental particles includes quarks, leptons and mediators who are the bosons mediating the basic interactions.

Quantum Chromo Dynamics (QCD) which is dynamics of strong interaction is characterised by Asymptotic Freedom and Confinement. Each quark can come into three colours-Red, Green and Blue in order to honour Pauli Exclusion Principle. Permanent confinement of quarks inside hadrons prevents us to observe a free quark- a consequence of the fact that all physical observable of states of nature are colour neutral.
Study of the structure of proton revealed that it is not a fundamental particle. So in our quest to ultimate structure of matter, we ended up with electrons and quarks (the constituent of proton and neutrons) which are elementary particles constituting an atom. The modern picture of atom can be seen in the picture above… electrons moving around and nucleus consists of quarks. This wonderful journey of Particle physics in search of the structure of atom from electrons to quark extends almost 75 years! First imagination of atom model came from Thomson and gradually we move forward with subsequent steps. In science each and every step is great either right or wrong as Newton said ‘If I have seen further than others, it is by standing upon the shoulders of giants’!!
The story does not end here. We have a long way to go. Proton is not as simple as consisting of three valence quarks only. Last picture shows its probable structure. It has a sea of virtual clouds consisting quark-anti quark pairs. As we probe smaller and smaller distances i.e. at higher energies, the structure seems to be more and more complicated. Till we know little about proton size, shape, mass, magnetic moment and spin. The size and shape of proton is yet to be determined which is very important as we know geometry depicts the interaction. Some theoretical investigations suggest that it can have fractal structure!!.The discovery by EMC collaboration indicates that constituent quarks appear to contribute very little to the proton spin which is termed as Proton spin crisis. Recent experiments suggest proton can have strange quark virtual sea in addition to up and down quarks and contribution from gluons, the mediator of quark-quark interaction. Deep inside proton may discover a new state of matter. In fact Proton may contain a universe inside!
Science is always an adventure, scientists love mysteries. This never ending journey is no less wonderful than that of journey of ‘Alice in Wonderland’. In the end let us remember famous quote by Rutherford himself ‘All of Physics is either impossible or trivial. It is impossible until you understand it, and then it becomes trivial’!!!
Till let journey continue….
Courtesy : Pictures from Google.
Visited 3198 times, 2 Visits today



