Introduction
Engineered nanoparticles (NPs) have broad applications in industry and medicine. Recently an era of nanotechnology has coined a new term “nanomedicine” which is the application of nanotechnology in medicine.1 The novelty of nanomedicine encompasses the smart delivery of nanosized (1-100 nm) therapeutic or diagnostic agents selectively to the diseased lesions and specifically elicit pharmacological effects without affecting the healthy cells and tissues. Thus, nanomedicine has been widely acclaimed as potential probes for the treatment, detection, and prevention of various diseases. Recently our world is passing through a perilous situation due to the sudden outburst of Coronavirus disease 2019 (COVID-19) caused by novel severe acute respiratory syndrome called coronavirus 2 (SARS-CoV-2)2. It is the need of an hour to develop novel therapeutics to combat this uncontrolled inflammation caused by the fulminant cytokine storm in recent COVID-19 infections.
Nanomedicine approach for Inflammatory diseases: A possible hope for COVID-19
To begin with an uncontrolled inflammation in COVID-19, we need to have an idea about inflammation which is simply the body’s own defense mechanism to any damage, making it an important part of the healing process.3 However, uncontrolled inflammation directly contributes to the pathogenesis of a diverse array of chronic diseases, such as infectious diseases, cancer, neurodegenerative disease, diabetes, cardiovascular disease, and immunometabolic disorders.4 Recently in the COVID-19 infection, it was observed that the certain cases of COVID-19 infections resulted in death by the propagation of the acute respiratory distress syndrome or ARDS. In ARDS, the lungs fill up with fluid preventing oxygenation and effective delivery of therapeutics through the inhalation route.5 It was observed that some patients suffering from COVID-19 has developed a cytokine storm4-5 i.e. an hyperinflammatory state is developed in their body triggered by viral infections. Today in the existing anti-inflammatory therapy, various steroidal or nonsteroidal anti-inflammatory drugs, corticosteroids have been broadly used for the treatment of different inflammatory diseases, despite having severe side effects.6 Biological therapeutics, such as monoclonal antibodies, cytokines have been used to treat such inflammatory diseases by interfering inflammation-associated molecular profiling and neutralizing disease-specific pro-inflammatory cytokines.7
During the last few years, specifically engineered nanoparticles (NPs) have earned emerging preferences over the use of conventional anti-inflammatory drugs due to their biodegradability, stealth characteristics, low toxicity and specific targeting efficacy with ensured better delivery of therapeutics with reduced side effects.8 Most popularly used NPs are polymeric nanoparticles, hydrogels, solid-lipid nanoparticles, liposomes, protein nanoparticles, metallic nanoparticles and dendrimers.9 A broad range of active moieties including antivirals, biologics, and nucleic acids can be loaded and delivered by these engineered nanocarriers. These nanocarriers have the potential of regulating the expression of pro and inflammatory molecules and targeting inflammatory sensors or macrophages through phagocytosis. These nanocarriers can specifically target to antigen presenting cells (APCs), could be of great value to promote cellular response or immune tolerance thanks to the surface tunability which allows them to passively (through size and surface charge of NPs) or actively (through decorating NPs with target molecules like specific antibodies) target these cells.10 These anti-inflammatory nanomedicines have shown excellent impacts in therapeutic development of rheumatoid arthritis (RA), diabetes, inflammatory bowel disease, asthma and Alzheimer’s disease. Thus, they could be used as potent probes in the treatment of hyper inflammatory COVID-19 infection.8-10 In the treatment of COVID-19, some researchers have already started using these anti-inflammatory nanomedicines to combat the deadly SARS-CoV-2 virus. Various well-known strategies such as the use of repurposed anti-inflammatory drugs (hydroxychloroquine, methotrexate) as the targets for RNA polymerase and the viral S protein with host receptor ACE2 have been applied as the major pathways of therapeutic development. Antibody targeting approach for surface S protein and for pro-inflammatory cytokines (IL-6/IL-6R) is also another popular strategy in the COVID-19 treatment.11-12 The reason for the selection of nanomedicines over conventional drugs was discussed in three main aspects and may be a sturdy way out to control over this COVID-19 pandemic.
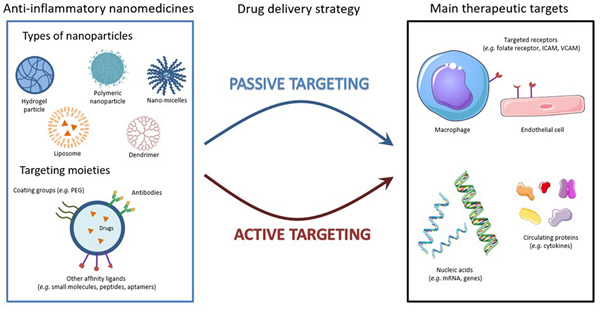
Figure 1. Anti-inflammatory nanomedicines: strategies and targets.8
Engineered nanocarrier as an alternative to conventional therapeutics
The major drawback of conventionally used anti-inflammatory drugs such as methotrexate (MTX), Curcumin (Cur) is their very low tolerable payloads. In order to solve this problem, in the treatment of RA, poly (lactic-co-glycolic acid) were integrated with gold (Au) nanoparticles and the RA targeting was achieved by tethering with arginine-glycine-aspartic acid (RGD) peptide. This nano vehicle was loaded with the anti-rheumatic drug MTX which displayed increased efficacy and reduced toxicity in CIA mice when compared to conventional treatments using MTX only.13 In addition, solid-lipid nanoparticles coated with CD44 receptor targeting hyaluronic acid (HA) has showed a significant improvement in collagen induced RA due to the selective accumulation of NPs in the inflamed tissues of mice.14 In inflammatory disease bowel syndrome, curcumin, a well-known anti-inflammatory drug is nanosized using some pH sensitive polymers to improve its bioavailability as well as targetability to the specified sites.14 The systematic circulation instability and the prerequisite of their intracellular delivery is the well-known limitation of nucleic acid (e.g., RNAi) drug candidates. Recently, a number of siRNA-encapsulated lipid-based nanoparticles (LNPs) were reported for the treatment of intractable diseases such as cancer, viral infection, inflammatory neurological disorder, and genetic diseases.15

Figure 2. Nanomedicine strategies for COVID-19 therapeutics development.12
Chemical engineering to therapeutics
Conventional drug molecules are altered to improve their compatibility with a particular class or type of nanocarriers, rendering this a more generic approach for drug candidates with similar physicochemical properties. The potent anti-inflammatory corticosteroid dexamethasone (Dex) nanoformulations have already been successfully used in different inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, liver fibrosis, wound healing and cancer. Most of these works have shown pH sensitive polymeric nanomedicines where Dex was attached to these polymers using biodegradable spacer molecules. This well-known anti-inflammatory Dex is extensively used in the recovery trail of COVID-19 treatments where patients are administered with the Dex daily showing the reduction in of COVID-19-related deaths by 35% in patients on the intensive care unit (ICU).16 Similarly, in another report, instead of using conventional drug hydroxy chloroquine, cholesterol-modified hydroxychloroquine (Cho-HCQ) loaded liposomes with reducing toxicity also inhibited the proliferation of rat lung fibroblasts, thereby, reducing pulmonary fibrosis.17 The proinflammatory cytokine IL-6 available from the cytokine storm of critically ill COVID-19 patients can be specifically targeted using anti-IL-6 antibody tocilizumab which has been an established therapy in the treatment of RA.18 Thus, similar to RA, these hyaluronate-gold nanoparticle entrapped Tocilizumab can be targeted to COVID-19 patients and may be an effective therapeutic strategy to fight the deadly virus.
Nanomedicine for Combination Drug Therapeutics.
Combination drug therapy is another accepted strategy for the treatment of COVID-19. There are several reports available on these nanoparticles modified hydrophobic/hydrophobic drug delivery for inflammatory diseases which can be potentially useful for this corona virus treatment. A nanosuspension of lipid nanoparticles (LNPs) loaded with three antiviral drugs (two hydrophobic: lopinavir and ritonavir and one hydrophilic: tenofovir) has been formulated to overcome the lymph node drug insufficiency of the oral combination of these drugs.19 In addition to the repurposed drug therapy, Patrick Couvreur et al. has also fabricated a multifunctional nanomedicine using bioconjugation strategy of adenosine to squalene with further formulation with tocopherol.20 It was shown that this nano formulation can target inflamed tissues in multiple murine models of inflammation for adenosine receptor activation and antioxidant action.
Conclusion
Advances in bio/nanotechnology and advanced nano/manufacturing coupled with open reporting and data sharing lay the foundation for rapid development of innovative therapeutic/vaccine technologies to make an impact during the COVID-19 pandemic. To date, there are no specific approved drugs for treating SARS-CoV-2, and vaccines are under clinical trials. The devastating effect of COVID-19 over the world may serve as an impetus for the scientific community, funding bodies, and stakeholders to commit more focused efforts toward development of platform technologies that encourage the preparedness for future pandemics. Along with rest of the world, in our country, some of the companies like Hetero drugs, Cipla Ltd, Zydus Cadila have started manufacturing and marketing some of the repurposed anti-inflammatory or anti-viral drugs remdesivir and hydroxychloroquine for the treatment of COVID-19 patients. All efforts are welcome to combat the virus, and nanotech-based approaches would bring a new perspective to conventional medicine for the inhibition of virus internalization or treatment. Certainly, in this era of advanced nanoscience, nanotechnology could play a frontline role in tackling this outbreak.
References
- Peer, J. M. Karp, S. Hong, et al. Nat. Nanotechnol. 2007, 2, 751.
- C. Walls, Y. J. Park, M. A. Tortorici, et al. Cell 2020, 180, 1.
- Nathan, Nature 2002, 420, 846.
- Molinaro, C. Boada, G.M. Del Rosal, et al. Cardiovasc. J. 2016, 12, 169.
- Impellizzeri, G. Bruschetta, Expert Opin. Emerg. Drugs 2015, 20, 75.
- R. Vane, R. M. Botting, Am. J. Med. 1998, 104, 2S.
- C. Taylor, M. Feldmann, Nat. Rev. Rheumatol. 2009, 5, 578.
- Brusini, M.Varna, et al. Adv. Drug Deliv Rev. 2020, https://doi.org/10.1016/j.addr.2020.07.010.
- G. Dacoba, A. Olivera, et al. Semin. Immunol. 2017, 34, 78.
- A. Petros, J. M. DeSimone, Nat. Rev. Drug Discov. 2010, 9, 615.
- Karimi, A. Ghasemi, P. S. Zangabad, et al. Chem. Soc. Rev. 2016, 45, 1457.
- Chauhan, M. J. Madou, et al. ACS Nano 2020, https://dx.doi.org/10.1021/acsnano.0c04006.
- H. Zhou, B. Qiu, et al. Cell. Physiol. Biochem. 2018, 47, 1207.
- Adams, A. Gonzalez-Duarte, et al. N. Engl. J. Med. 2018, 379, 11.
- Lammers, A.M. Sofias, et al. Nat. Nanotechnol, 2020, 6, 618.
- A. Kulkarni, A. N. Bade, et al. Prodrug. Nat. Mater. 2020, DOI: 10.1038/s41563-020-0674.
- Liu, J. Ren, Z. He, et al. Sci. Rep. 2017, 7, 10737.
- Lee, M.Y. Lee, et al. ACS Nano. 2014, 8, 4790.
- C. Kraft, L. A. McConnachie, et al. J. Control. Rel. 2018, 275, 229−241.
- Dormont, R. Brusini, C. Cailleau et al. Sci. Adv. 2020, 6, 5466.
Visited 1706 times, 1 Visit today



