Introduction: Importance of x-ray in 20th century Human civilization
Discovery of ‘x-ray’ is one of the significant factors to enhance the quality of modern science and life. It enters into realm of the practical use in medical physics instantly after berth. X-ray is widely used in physical, chemical, biological science and clinical laboratory. It is noteworthy to mention that x-ray opens the many interesting doors of new science. Use of physics based techniques and instruments in medical science are very important. However, in this pandemic situation due to the spread of COVID-19 virus it becomes a centre of discussion. Although most basic research has been suspended by the coronavirus pandemic, some labs remain open to engage in a furious effort to find treatments for the disease. Physicists and chemists are vital to a key part of that quest: decoding the three-dimensional structures of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins and finding locations where drugs could latch on and disable the viral machinery. It is noteworthy to mention that physics-based techniques play a huge role in the field of structural biology. And it has long history and relation with the x-ray crystallography and x-ray production technique. The vast majority of biological macromolecule structures are obtained by X-ray crystallography, going back to 1934, when John Desmond Bernal and Dorothy Hodgkin recorded the first X-ray diffraction pattern of a crystallized protein, the digestive enzyme pepsin. Their work stemmed from that of physicists such as Wilhelm Röntgen, who discovered X-rays; Max von Laue, who discovered that X-ray wavelengths are comparable with inter-atomic distances and are therefore diffracted by crystals; and William Henry and William Lawrence Bragg, who showed how to use a diffraction pattern to analyse the corresponding crystal structure. Hodgkin went on to win the 1964 Nobel Prize for Chemistry for her determinations by X-ray techniques of the structures of important biochemical substances.
Nobel Prizes awarded for Research in x-ray and discovery using x-ray
The importance of x-ray in human civilization may be understood little by the awarding of Nobel Prized in the field of “X-ray”.
- Sir Wilhelm Conrad Roentgen obtained “Nobel Prize” for his remarkable discovery of x-ray in 1901.
- In 1902 Endrick A.Lorentz & Pieter Zeeman were awarded Nobel Prize in recognition of extraordinary services; they have done research into influence of magnetism upon radiation phenomena.
- Another research and discovery which causes a paradigm shift in the field of physical, chemical and biological science is x-ray crystallographic diffraction.
- In 1914 Max Von Laue was awarded Nobel Prize for his discovery of diffraction of x rays by crystals.
- William Henry Bragg & William Lawrence Bragg were awarded Nobel Prize in 1915 for their services in analysis of crystal structure by means of X-rays.
- Charles Glover Barkla is remembered and awarded Nobel Prize in 1917 for his contribution in research of characteristic roentgen radiation of elements.
- Karl Manne Georg Siegbahn obtained Nobel Prize for his discoveries and research in field of X ray spectroscopy in 1924.
- In 1927 Arthur holly Compton was awarded Nobel Prize for his contributions to the knowledge of molecular structure through his investigations on dipole moments & on the Diffraction of x rays and electrons in gases.
- McLeod Cormack & G. Newbold Hounsfield were awarded Nobel Prize in 1979 for the development of computed axial tomography.
- In 1981 Kai M.Siegbahn were awarded Nobel Prize for his contribution to the development of high resolution electron microscopy.
- Herbert A.Hauptman & Jerome Karle were obtained Nobel Prize on 1985 for their outstanding Achievements in development of direct methods for the determination of Crystal Structures.
- In 2002 Raymond Davis Jr., Masatoshi Koshiba, Riccardo Giacconi were awarded Nobel prize for pioneering contributions to astrophysics, in particular for detection of cosmic neutrinos and discovery of cosmic x ray sources.
Production of x-ray at the early stage:
X-ray is an electromagnetic wave. The wave length range is 0.01 to 10nm. German physicist Wilhelm Röntgen is credited for discover of x-rays in 1895. However, x-ray emitting from crookes tubes and experimental discharge tubes was invented around 1875 but it was first systematic studied by the physicist Röntgen. Dr. Röntgen received the first Nobel Prize in Physics in 1901 “in recognition of the extraordinary services he has rendered by the discovery of the remarkable rays subsequently named after him”. Primarily x-ray was produced in crookes tube. But the tube is unreliable as the small quantity gas is absorbed by wall of the tube itself. X-ray production method was upgraded due to the invention of the thermionic diode by John Ambrose Fleming on 1904. Nobel Prize received by Charles Barkla in 1917 “For his discovery of the characteristic Röntgen radiation of the elements”, another important step in the development of X-ray spectroscopy. Method of the x-ray production crossed another step by invention of Coolidge x-ray tube during 1912 by Willam D. Coolidge for continuous x-ray emission and used for a long time.

Diffractometer is used in laboratory for structural analysis.
Paradigm shift in X-Ray production:
As the x-ray is electromagnetic wave, shows phenomena like other light or electromagnetic wave viz. reflection, diffraction, absorption etc. Von Laue in 1912 discovers the diffraction of x-ray by crystals, building block of the solid material and emerge a new field ‘X-ray Crystallography’. It helps the scientist to look inside a solid and reveal the internal structure and symmetry of the materials.
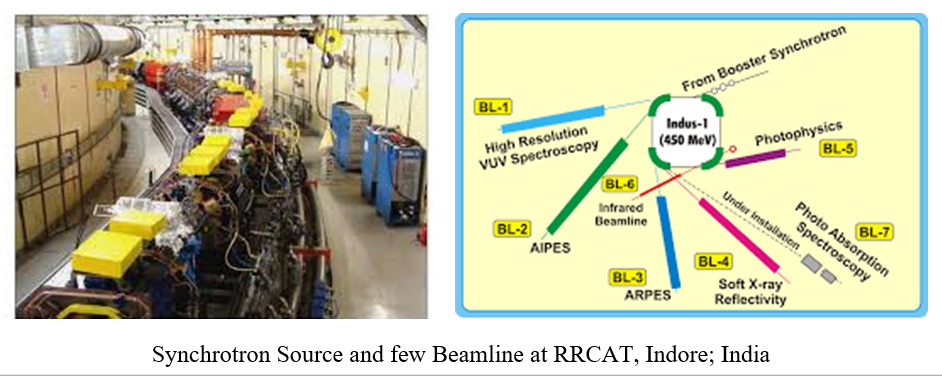
However, x-ray imagining is the phenomena of x-ray absorption by the material and widely known to us in the field of clinical medicine. Another contribution of x-ray in the development of material science is X-ray Absorption Fine Spectroscopy (XAFS). It is one of the wide spread application of the x-ray in the scientific purpose, awaited a decade after discovery of x-ray diffraction. XAFS is the modern development of X-ray Absorption Spectroscopy (XAS). XAFS require a powerful stable x-ray source and the demand meets by the electron synchrotron with stable and brilliant storage rings (There is only one synchrotron in India at RRCAT, Indore). It also further improved the field of x-ray diffraction of the material due to high penetration depth and directionality. Synchrotron x-rays were first observed in 1947. It is note-worthy to mention that, the structure of DNA was discovered by J. Watson and F. Crick using the x-ray in 1953.
Structural Biology – Virus: Potential Contribution of Synchrotron to overcome Pandemic:
Single biological molecules diffract X-rays, but only very weakly. Crystallization, as Bernal and Hodgkin employed for pepsin, is helpful because it results in the repetition of huge numbers of molecules in an ordered, 3D lattice, so that all their tiny signals reinforce one another and become detectable – by photographic plates in the early days and by active pixel detectors today. These signals are not images of the molecules, for there are no materials that can substantially refract, and thereby focus, scattered X-rays. Rather, the signals are merely the sum of the contributions of X-rays diffracted from different parts of the molecule. To pick apart these contributions, structural biologists rely on a mathematical tool – the Fourier transform. The calculated contributions are then equated with possible atomic structures by a lot of careful (and now largely computer-driven) interpretation. For structural biologists, x-ray crystallography is by far the most commonly used tool for unravelling protein structures. It also produces the highest resolution images of structures. The process typically is performed at cryogenic temperatures to limit ionizing- radiation damage to proteins. Small-angle X-Ray scattering has dedicated beamlines at each of the synchrotron light sources. The technique lacks the angular resolution of crystallography, but it can be used to examine macromolecules in solution, nearer a protein’s native state at room temperature. The scattering can explore how structures change over time as the virus matures. That evolutionary process could occur over hours or days, says Britt Hedman, SSRL science director.
Role of high energetic x-ray beam
Here the interactive signal is X-rays. Nowadays, synchrotron radiation sources are ideal for macromolecular crystallography because they produce high-intensity X-rays with a very narrow spread of wavelengths. This tremendous job is done by the synchrotron source within just a second where as traditional x-ray diffractor machine take time around a month. The four-year delay in obtaining the structure of HIV protease was primarily due not to the brilliance or quality of X-rays, but to the lack of sizeable crystals of the enzyme. Current synchrotrons and ever newer free-electron lasers – which extract diffraction data from molecular crystals in the few femtoseconds before they are annihilated – employ techniques such as serial crystallography to build up a complete diffraction dataset from numerous partial datasets of crystals that would otherwise be too small. More¬over, both the crystallization and data collection are now automated, so that structural biologists need not even visit a light source themselves: they simply post their proteins to a facility and download the dataset when it is ready.
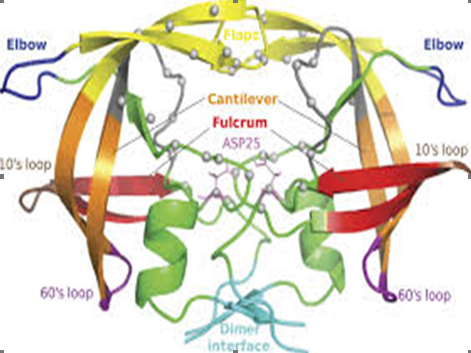
HIV protease
As of early April, the life sciences beam lines were open for SARS-CoV-2 research at the UK’s Diamond Light Source. In the US, at least 23 groups were working at the Advanced Photon Source at Argonne National Laboratory as of press time, according to Bob Fischetti, life sciences adviser at the APS. The National Synchrotron Light Source II at Brookhaven National Laboratory, SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL), and the Advanced Light Source at Lawrence Berkeley National Laboratory are operating with minimal staff, each keeping open several x-ray protein crystallography beamlines strictly for corona virus research. The BESSY II light source in Berlin closed briefly but resumed operations on 2 April for coronavirus research, which is also ongoing at the Shanghai synchrotron in China, where the first 3D structure of the main protease protein was resolved. Officials there did not respond to requests for comment. The European Synchrotron Radiation Facility in France has been closed for an upgrade, but it announced in early April that it would consider reopening beamlines on a case-by case basis for coronavirus research.

Macromolecular Crystallographic Beamline at Diamond Light Source; UK
World wide there is resurgence to uncover SARS-CoV-2 structure and get rid of this pandemic. On 5 February this year, a little over a month after the Chinese authorities disclosed the existence of the new coronavirus, a research team led by Zihe Rao and Haitao Yang at ShanghaiTech University in China uploaded the structure of the virus’s main protease to the Protein Data Bank, having obtained the dataset using X-ray crystallography at the Shanghai Synchrotron Radiation Facility. “A decade ago, that would have taken a year,” says Wlodawer. The structure is already helping pharmaceutical companies to explore potential drugs, such as those used to tackle HIV.
References:
- “World’s physics instruments turn their focus to COVID-19” Physics Today 73, 5, 22 (2020) by David Kramer.
- “Brief history of X-ray tube patents” World Patent Information 37, 48(2014) by Marcio Luis Ferreira Nascimento.
- “Early history of X rays.” Beam Line 25, 10(1995) by A. Assmus.
Visited 1169 times, 2 Visits today



